The Active Principles of the
Seeds of Rivea Corymbosa and Ipomoea Violacea
By Albert Hofmann, 1964
 Ololiuhqui
Ololiuhqui (
Rivea Corymbosa) is a Mexican divinatory agent. This article will consider the chemical investigations that led to the isolation of the active principles of this old Aztec magic drug and to the elucidation of its chemical structure.
The road that led to the discovery of the active principles of
ololiuhqui is both remarkable and significant. I will, therefore, preface the chemical report with a short account of the background and results of these investigations.
It all started exactly 20 years ago, when I was engaged in the synthesis of lysergic acid derivatives in the pharmaceutical-chemical research laboratory of Sandoz Ltd. in Basel, Switzerland.
Lysergic acid is the foundation stone of the ergot alkaloids, the active principles of the fungus-product ergot. Botanically speaking, ergot is the scierotia of the filamentous fungus Claviceps purpurea which grows on grasses, especially rye. The ears of rye that have been attacked by the fungus develop into long, dark pegs to form ergot.
The chemical and pharmacological investigation of the ergot alkaloids has been a mein field of research of the natural products department of the Sandoz laboratories since the discovery of ergotamine by A. Stoll in 1918. These investigations have resulted in a variety of useful pharmaceuticals which find wide application in obstetrics, in internal medicine, in neurology and psychiatry.
On the 16th of April 1943, upon recrystallizing d-lysergic acid diethylamide tartrate, which I had produced from natural lysergic acid and diethylamine by way of the lysergic acid hydrazide and azide, I suddenly became strangely inebriated. The external world became changed as in a dream. Objects appeared to gain in relief ; they assumed unusual dimensions; and colors became more glowing. Even self-perception and the sense of time were changed. When the eyes were closed, colored pictures flashed past in a quickly changing kaleidoscope.
After a few hours, the not unpleasant inebriation, which had been experienced whilst I was fully conscious, disappeared. What had caused this condition? Subsequent systematic self-experimentation with the chemicals that I had used on that day were to provide the answer. Lysergic acid diethylamide was tested, amongst other substances, as it was possible that a drop had fallen on my fingers and had been absorbed by the skin.
I commenced my experiments on this compound by taking 0.5 ml of a 0.5 per mille aqueous solution, corresponding to 0.25 mg of d-lysergic acid diethylamide tartrate. This extremely small quantity proved to be a substantial overdose. A state of inebriation followed, lasting for a number of hours and filled with dramatic experiences, which have been described in former publications.
[1,2]
This is how the most active psychotomimetic, hallucinogenic compound known up to the present was discovered, a compound which subsequently attained great importance under the name of LSD 25 (Delysid
R) in experimental psychiatry and, recently, in psychotherapy as well.
Lysergic acid diethylamide (formula I) was produced during the course of large-scale investigations on semi-synthetic amides of lysergic acid, after d-lysergic acid L-propanolamide-(2) (formula II), which was found to be identical with the natural alkaloid ergometrine ( also known as ergonovine), had been synthesized. This was the first synthesis of a natural ergot alkaloid.
[3]
After the discovery of the psychotomimetic activity of LSD, a great number of further simple lysergic acid amides were synthesized in our laboratories
[4] so as to ascertain the relationship between chemical structure and psychic activity in this group of compounds. The unsubstituted d-lysergic acid amide (=ergine) ( formula III) and the d-isolysergic acid amide (=isoergine) (formula IV), were amongst these semi-synthetic analogues of LSD. Ergine, isoergine and ergometrine were later found to be active principles of ololiuhqui, as will be shown below.
The discovery of LSD and subsequent research in the field of psychotomimetics caused the Mexican fungi to be brought to our laboratories. The preceding article told the history of the discovery of these fungi and the contribution to it by engineer Robert J. Weitlaner and his daughter Irmgard Weitlaner-Johnson, the work of Reko and of Schultes, as well as their rediscovery by the husband and wife team of Valentina P. and R. Gordon Wasson in collaboration with the mycologist Professor Roger Heim.
After chemical analysis in a Paris laboratory had proved unsuccessful, Professor Heim sent a few of the hallucinogenic fungi to us in Basel on the assumption that the necessary conditions for a successful chemical investigation would be present in the laboratory in which LSD was discovered. During the course of chemical studies on
teonanicatl, psilocybin and psilocin were discovered as active principles of the most important hallucinogenic fungi.
[5]
Thus it was that the present investigations were crowned with success within an unusually short time, since these two active principles are indole compounds that are structurally related to LSD and ergot alkaloids. In the chain of events that led to the ololiuhqui problem, the most important factor was that the writer came into personal contact with Wasson as a result of investigations into the active principles of
teonanicatl.
Fired by the discussions with this outstanding expert on the Mexican magic drugs and encouraged by our successes with the hallucinogenic fungi, we decided to tackle the chemical investigation of the third most important Mexican psychotornimetic after "peyotl" and "teonanicatl"—namely "ololiuhqui". With the help of Wasson, we obtained authentic "ololiuhqui", as he sent us two samples from his expedition in Mexico in the late summer of 1959. With the samples, he wrote from Mexico City on August 6, 1959, the following:
"I am sending you... a small parcel of seeds that I take to be Rivea corymbosa, otherwise known as ololiuhqui, well known narcotic of the Aztecs, called in Huautla, la semilla de la Virgen. This parcel, you will find, consists of two little bottles, which represent two deliveries of seeds made to us in Huautla, and a larger batch of seeds, delivered to us by Francisco Ortega (Chico), the Zapotec guide, who himself gathered the seeds from the plants at the Zapotec town of San Bartolo Yautepec."
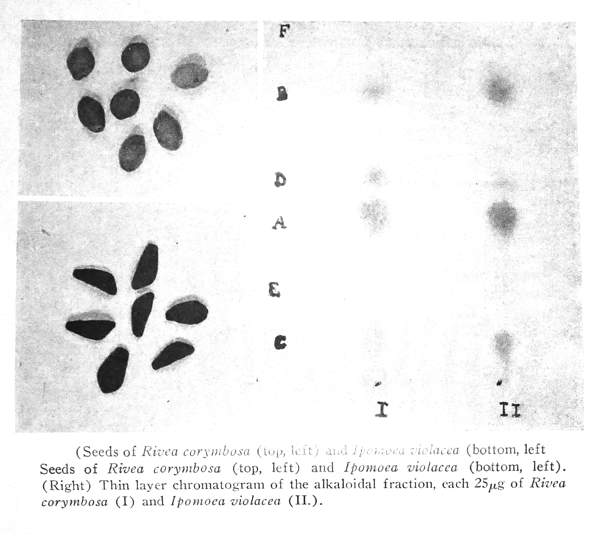
The first-mentioned light brown, roundish seeds (see Plate IX, top left) from Huautla (21 g), upon botanical investigation, were found to be Rivea corymbosa (L). Ha11.f., whilst the black and angular seeds (see Plate IX, bottom left) were found to represent Ipomoea violacea L.(204 g).
The first small samples were sufficient for a number of chemical-analytical experiments, which showed the presence of indole compounds. This interesting result induced us to order greater quantities of these two seeds from Wasson. This second, large contingent of seeds (12 kg of seeds of Rivea corymbosa and 14 kg of Ipomoea violacea) was obtained with the aid of the Weitlaners, about whom Wasson gave the following information to the writer in a letter of 10th December 1959:
"Robert Weitlaner is an Austrian, a naturalized Mexican citizen... He is a field Anthropologist and likes being in the field much better than lecturing to the students in the Instituto Nacional de Antropologia e Historia, where he has a post. He is past 70 already, but still goes out for months at a time with almost no luggage, living in the villages. Irmgard is his daughter, the native-textile expert of the Museo Nacional..."
These Rivea seeds obtained with the aid of the Weitlaners were gathered in the vicinity of Ocozocoautla (Chiapas), the Ipomoea seeds in the Zapotec region by Thomas MacDougall and Francisco Ortega.
In 1960, MacDougall published his important discovery that, especially in the region of the Zapotecs, the seeds of a second twining species, which he found to be Ipomoea violacea, were used in conjunction with or instead of ololiuhqui.
[6]
By using the large quantities of seeds of Rivea corymbosa and Ipomoea violacea, which we received in the early part of 1960 in the manner already described, we were able to isolate the main active principles and identify these chemically during the course of the summer. This isolation and identification will be reported in detail below. In a number of ways, the results of these investigations were surprising.
The active principles of the Mexican morning glory drugs proved to be ergot alkaloids. The two main components were, in the case of both seeds, d-lysergic acid amide (ergine) and disolysergic acid amide (isoergine), whilst four additional alkaloids were present. The former are closely related to d-lysergic acid diethylamide (LSD), which we had, as has already been mentioned, produced synthetically and investigated many years before. From the phytochemical point of view, this finding was unexpected and of particular interest, because lysergic acid alkaloids, which had hitherto been found only in the lower fungi in the genus Claviceps, were now, for the first time, indicated for the higher plants, in the phanerogamic family Canvolvidaceae.
The isolation of lysergic acid amides from ololiuhqui closed what is in reality a most strangely coincidental circle of research.
It was with the discovery of lysergic acid diethylamide (LSD) as a highly active psychotomimetic agent, during investigations on simple lysergic acid amides, that our research in the field of hallucinogenic compounds commenced.
It was within the framework of this activity that the sacred Mexican fungi came to our laboratories. It was during the course of these investigations that I made personal contact with Wasson. And it was as a result of this contact that the investigations of ololiuhqui were conducted. In this sacred drug, lysergic acid amides, which made their appearance in the initial stages of our psychotomimetic research, were again found as active principles.
FORMER INVESTIGATIONS ON OLOLIUHQUI
In the classical study of the oloiuhqui problem by R. E. Schultes, published in 1941
[7] (in which the historical, ethnographical and taxonomical aspects are treated in an excellent manner), there is discussed the only chemical investigation that had been done on the active principles of the seeds of Rivea corymbosa before the studies carried out by us. It was carried out by the pharmacologist, C. G. Santesson, in Stockholm in 1937.
[8] He was, however, unsuccessful in isolating defined, crystallized compounds. Certain reactions seemed to suggest the presence of gluco-alkaloids.
Following Schultes' work, only two original publications have appeared that deal with the psychic action of ololiuhqui seeds on volunteers. In 1955, a Canadian psychiatrist, H. Osmond, conducted a series of experiments on himself. After taking 60 to 100 Rivea seeds, he passed into a state of apathy and listlessness accompanied by increased visual sensitivity. After about four hours, there followed a period in which he had a relaxed feeling of well-being, a feeling that lasted for some time.
[9] In contrast to this result, V. J. Kinross-Wright in 1958 published the results of experiments performed on eight male volunteers who had taken doses of up to 125 seeds administered without any ascertainable effect in a single case.
[10]
PLATE VIII
Isolation and chemical identification of the active
alkaloidal principles
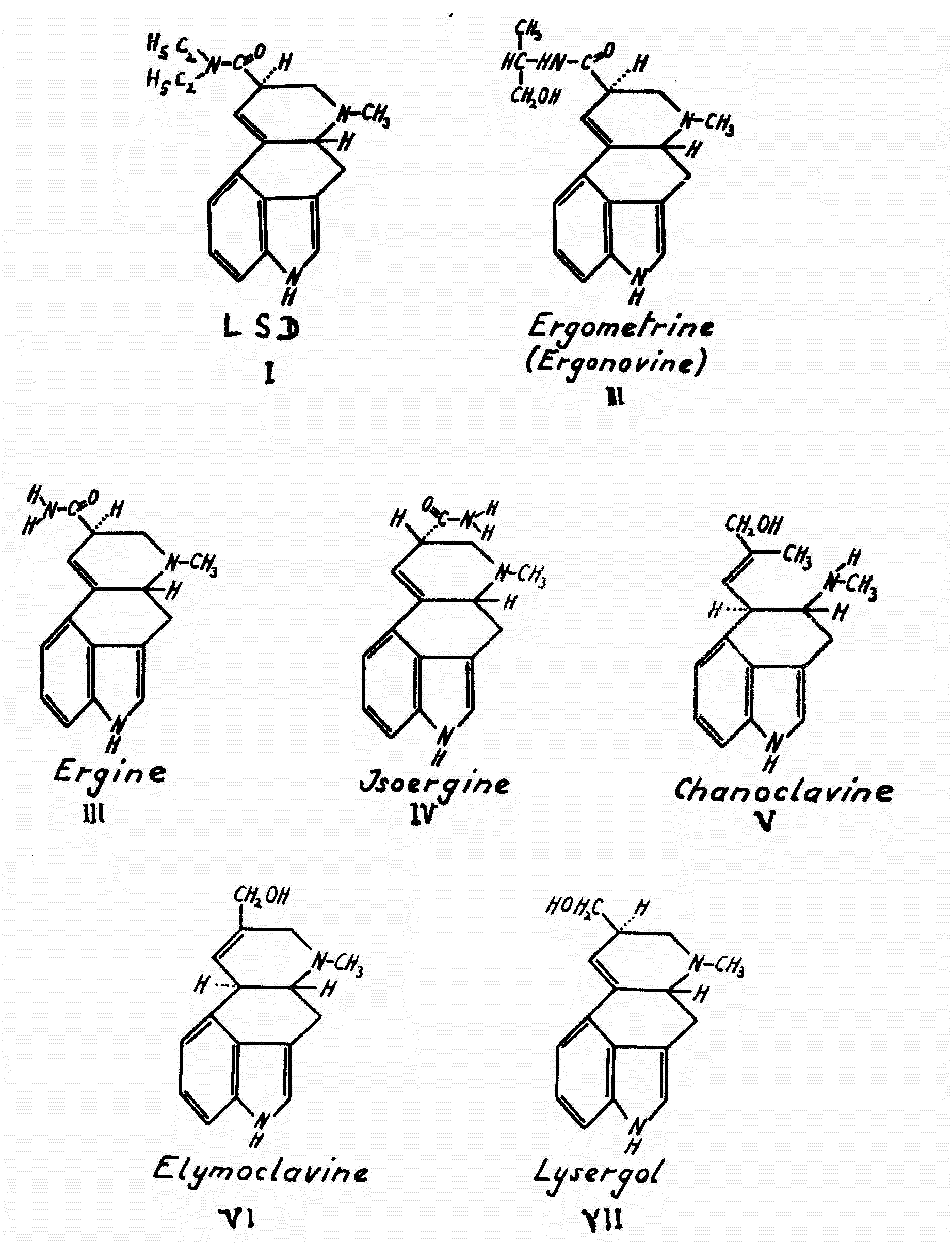 Plate IX
Plate IX
This plate shows the seeds of Rivea corymbosa (L.) Hall. f. and of I pomoea violacea L., the origin of which has been given above and which were used for the chemical investigations now described. Plate VIII shows plants in bloom that were cultivated from these seeds:
We started our extraction studies with Rivea corymbosa. Since we knew nothing of the chemical nature and sensitivity of the active principles, only neutral solvents were used and all extracts were evaporated carefully at low temperature. The finely powdered seeds were extracted with methanol, and the evaporated methanol extracts were defatted with petroleum ether.
The defatted residue was tested for various kinds of alkaloids, especially for indolic compounds, since the indole structure was known to occur in psychotomimetic agents. Indeed, when paper chromatograms of this Rivea extract were developed by spraying with a benzene solution of p-dimethylamino benzaldehyde and subsequently treated with hydrochloric acid gas, violet-blue spots appeared, indicating the presence of indolic compounds. In order to assess whether this indole fraction actually represented the active principle, we collected some milligrams of this fraction from a great number of paper chromatograms, and my laboratory assistant H. Tscherter and I tested it on ourselves.
After my experience with LSD, I have become cautious: we started by taking doses as small as 0.1 mg, gradually increasing the dosage. With 2 mg of this crude indole fraction we got clear-cut psychic effects: a dream-like state resulted with drowsiness and alterations in the perception of objects and colors. This showed that the indole fraction of the Rivea extract contained the psychic active principles.
The paper chromatographical testing of the extract of I ponwea violacea showed that here, too, the same or a similar indole compound was present. An even better separation than by the paper chromatogram was attained by thin layer chromatography. In Plate IX, right, the chromatograms of the extracts of Rivea corymbosa a7d I pomoea violacea, which were obtained on plates with aluminum oxide layer, using chloroform containing 5% of methanol as the moving phase, are shown side by side. The indole compounds were made visible by spraying with a 5% solution of p-dimethylamino benzaldehyde in concentrated hydrochloric acid and treating with the fumes of aqua regia.
[[PLATE IX]]
Rivea Corymbosa and Ipomoea Violacea
When larger quantities of seeds of Rivea corymbosa and 1 pomoea violacea were available, the indole compounds could be obtained in preparative quantities. It was found that they were alkaloidal in nature and that they could be isolated by the usual methods used for the extraction and purification of alkaloids. For this purpose, the finely ground seeds were made alkaline with sodium bicarbonate, then extracted with ethyl acetate.
The alkaloids were then removed from the extracts, which had been concentrated to a small volume in vacuum, with aqueous tartaric acid from which they were again shaken with ethyl acetate after making the mixture alkaline with a sodium bicarbonate solution. From the alkaloid fractions thus obtained, the individual components visible in the thin layer chromatogram could be separated by fractional crystallization, chromatography on aluminum oxide columns and thin layer plates with aluminum oxide and silica gel layers, on a preparative scale. The separated compounds were obtained in crystalline form and could be identified chemically. For further details, the reader is referred to our chemical publications.
[11,12,13,14] Only the results of the chemical investigations can be summarized briefly within the scope of this article. These are given in Table I.
The fact that Ipomoea violacea contains a greater total of active principles than does Rivea corymbosa explains why the Indians used smaller quantities of badoh negro (Ipomoea) than of badoh (Rivea).
Identification of the individual indole bases showed that ergot alkaloids were present.
The main component of the alkaloid mixture in the Rivea and Ipomoea seeds, which corresponds to spot A, is d-lysergic acid amide (ergine) ( formula III), a compound that was first obtained as a cleavage product upon alkaline hydrolysis of ergot alkaloids'
[15] and then also by partial synthesis from lysergic acid and recently as a genuine alkaloid from the ergot of Paspalum grass.
[16] The alkaloid corresponding to spot B in the chromatogram was found to be identical with d-i3olysergic acid amide (isoergine) (formula IV) which, as in the case of ergine, was already known as the hydrolysis product of ergot alkaloids
[17] and as a natural alkaloid.
The third alkaloid, chanoclavine (formula V), which forms spot C in the chromatogram, had been discovered by us in ergot of the tropical millet cob Pennisetum typhoideurn.
[18] Elymoclavine (formula VI), contained in spot D, was first isolated from the ergot of the wild grass Elymusmollis.
[19]
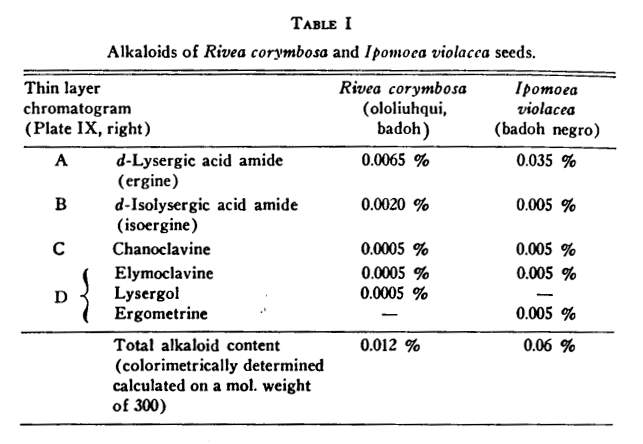
Ergometrine (formula II), the alkaloid which is mainly responsible for the uterotonic hemostatic action of the ergot drug, could only recently be identified as one of the active principles of Ipomoea violacea."
[20] Together with elymoclavine, it forms spot D in the thin layer chromatogram (Plate IX, right). The seeds of Rivea corymbosa either do not contain this compound or else only traces thereof. Instead, we found lysergol (formula VII) in the last mentioned seed, an alkaloid absent from the seeds of Ipomoea violacea. Lysergol was produced synthetically
[21] in our laboratories (as were d-lysergic acid amide, d-isolysergic acid amide and ergometrine) before it was discovered as one of the active principles of a Mexican magic drug. The compounds corresponding to spots E and F are present in such small quantities that they have hitherto not been identified.
In Plate VII, the structural formulas of the six alkaloids now isolated from ololiuhqui and badoh negro are depicted. These formulas clearly show the close relationship between ololiuhqui's active principles and the most active hallucinogenic agent known thus far, the synthetically produced LSD (formula I).
As has already been pointed out, the discovery of ergot alkaloids in the higher plants is a most unexpected phytochemical discovery. In view of the uniqueness of these findings, other investigators found it necessary to ascertain whether these alkaloids were actually produced by plant tissue or whether they were produced by fungi or bacteria present in the seeds. Before publishing our results, we examined our seed samples for attack by fungus and found that they were healthy and had not been infected. Furthermore, we had detected the alkaloids in fresh leaves, stalks and roots of Ipomoea violacea and, to a very small extent also, in the leaves of Rivea corymbosa.
[20] These were results that showed that ergot alkaloids were in fact produced by tissues of Rivea corymbosa and Ipomoea violacea and not by fungi infecting the seeds.
Our results were confirmed by the detailed investigations of W. A. Taber and R. A. Heacock who ascertained that the alkaloids are concentrated in the embryo of the seeds and are absent from the shells that had occasionally been attacked by fungi.
[22] The occurrence of small quantities of alkaloids in the leaves and stems of Rivea corymbosa was also confirmed.
[23] W. A. Taber, L. C. Vining and R. A. Heacock then also investigated the seeds of a number of commercially available varieties of Morning Glory (Ipomoea and Convolvidus spp.) and were able to trace the presence of alkaloids in a number of these ornamental plants.
[24] The quantitative determination and the identification of clavine and lysergic acid alkaloids, however, were done only colorimetrically or by means of paper and thin layer chromatography. In no instance were the individual alkaloids isolated and crystallized by the authors.
PHARMACOLOGICAL AND CLINICAL ACTIVITY OF THE ISOLATED ALKALOIDS.
There is no doubt that the alkaloids isolated from the seeds of Rivea corymbosa and Ipomoea violacea are the active principles of these magic plants. Aside from the described alkaloids, a large quantity of a new glucoside, which was named turbicoryn by M. C. Perezamador and J. Herrin, was isolated from the seeds of Rivea corymbosa.
[25,26] It is most improbable that the presence of this glucoside has anything to do with the psychotomimetic action of ololiuhqui as, according to our observations, the seeds of Ipomoea violacea, which are stronger than the Rivea seeds, contain none of this glucoside or only small traces of it. On the other hand, the high pharmacological and psychic activity of the lysergic acid amides, as well as of elymoclavine and lysergol, is certain.
D-lysergic acid amide (designation of compound undergoing tests: LA 111) was tested pharmacologically and clinically during the course of investigations on d-lysergic acid diethylamide (LSD 25) and related compounds long before it was known to be a component of ololiuhqui. Already at that stage we had ascertained in experiments on ourselves, a psychotomimetic activity with a marked narcotic component with dosages of 0.5 to 1 mg.
The following paragraph is taken from a hitherto unpublished record of the first experiment which the writer performed upon himself with LA 111 on October 30th, 1947.
10.00 h: Intramuscular injection of 0.5 ml of 1 per mille solution of LA 111 (=0.5 mg d-lysergic acid amide).
11.00 h: Tiredness in the neck, slight nausea.
11:05 h: Tired, dreamy, incapable of clear thoughts. Very sensitive to noises which give an unpleasant sensation.
11.10 h Desire to lie down and sleep. Genuine physical and mental tiredness, which is not experienced as an unpleasant sensation. Slept for 3 hours.
15.00 h: Return of normal condition with full capacity for performing work.
This action of d-lysergic acid amide was later confirmed by the comparative systematic investigation of H. Solms.
[27,28] He describes the action as follows: LA 111 induces indifference, a decrease in psychomotor activity, the feeling of sinking into nothingness and a desire to sleep until finally an increased clouding of consciousness does produce sleep.
Clinical investigations have been initiated with d-isolysergic acid amide, but no results are available yet. Upon taking 2 mg of isoergine himself, the writer experienced tiredness, apathy, a feeling of mental emptiness and of the unreality and complete meaninglessness of the outside world.
Elymoclavine and lysergol elicit an excitation syndrome in various animals that is caused by a central stimulation of the sympathicus.
[29] The results of clinical testing are not, as yet, available.
Psychotomimetic effects are unknown for ergometrine, which is used to a large extent in obstetrics as a uterotonic and hemostatic agent. When using the small dosages which are administered for this purpose, the alkaloid apparently has no action on the psychic functions. Its occurrence in the alkaloid mixture of Ipomoea violacea can thus have no significant effect on the action of badoh negro.
Furthermore, chanoclavine, which has no outstanding pharmacological activity, appears to play no part in the occurrence of the psychic effects of badoh and badoh negro.
According to the results of experiments performed thus far with pure alkaloids, it appears as though d-lysergic acid amide,
elyinoclavine and lysergol and possibly also d-isolysergic acid amide are mainly responsible for the psychic effect of ololiuhqui.
Systematic comparative investigations are presently being performed with the pure alkaloidal principles of ololiuhqui and total extracts from the seeds so as to ascertain the psychic effect onhumans. These will show whether the alkaloids described are alone responsible for the psychotomimetic effects (which, in view of our present knowledge, seems probable) or whether other factors play a part.
FOOTNOTES
- The History of LSD 25. "Triangel". Sandoz Journal of Medical Science 2:117 (1955).
- A. Hofmann : Psychotomimetic Drugs. Chemical and Pharmacological Aspects. Acta Physiol. Pharmcol. Neerlandica 8:240 (1959).
- A. Stoll und A. Hofmann : Partialsynthese von Alkaloiden vom Typus des Ergobasins. Hely. Chim. Acta 26:944 (1943).
- A. Stoll und A. Hofmann : Amide der stereoisomeren Lysergsatiren und Dihydro-lysergsauren, Hely. Chim. Acta 38: 421 (1955).
- A. Hofmann, R. Heim, A. Brack, H. Kobel, A. Frey, H. Ott, Th. Petrzilka und F. Troxler : Psilocybin und Psilocin, zwei psychotrope Wirkstoffe aus mexikanischen Rauschpilzen. Hely. Chim. Acta 42: 1557 (1959).
- Th. MacDougall : Ipomoea tricolor, a Hallucinogenic Plant of the Zapotecs. Boletin del Centro de Investigaciones Antropologicas de Mexico, No. 6, March 1 (1960).
- R. E. Schultes : A Contribution to Our Knowledge of Rivea corymbosa, the Narcotic Ololiuqui of the Aztecs. Botanical Museum of Harvard University, Cambridge, Massachusetts (1941).
- C. G. Santesson : Notiz iiber Piule, eine mexikanische Rauschdroge. Ethnol. Stud. 4: 1 (1937), Gothenburg; Arch. Pharm. und Ber. Deutsch. Pharm. Ges. 1937, 532.
- H. Osmond : Ololiuqui : The Ancient Aztec Narcotic. J. of Mental Science 101: 526 (1955).
- V. J. Kinross-Wright : Research on Ololiuqui: The Aztec Drug. Proc. 1st Internat. Cong. Neuro-Pharmacol., Rome (1958). In: Neuro-Psychopharmacology. Ed. by P. B. Bradley, P. Deniker und C. Radouco-Thomas ; Elsevier Publ. Co., Amsterdam-London-New York-Princeton (1959) p. 453.
- A. Hofmann und H. Tscherter : Isolierung von Lysergsaure-Alkaloiden aus der mexikanischen Zauberdroge Ololiuqui (Rivea corymbosa (L.) Hall.f.), Experientia 16: 414 (1960).
- A. Hofmann, "Hallucinogenic Principles of Ololiuhqui," paper read at the International Symposium on the Chemistry of Natural Products, Melbourne, August 18 (1960).
- A. Hofmann: Die Wirkstoffe der mexikanschen Zauberdroge Ololiuqui. Planta Medica 9: 354 (1961).
- A. Hofmann und A. Cerletti : Die Wirkstoffe der dritten aztekischen Zauberdroge. Deutsche Med. Wschr. 86 : 885 (1961).
- S. Smith and G. M. Timmis : The Alkaloids of Ergot. Part VII. isoErgine and isoLysergic Acids. J. Chem. Soc. 1936: 1440.
- F. Arcamone, C. Bonin, E. B. Chain, A. Ferretti, P Penella, A. Tonolo and L. Vero: Production of Lysergic acid derivatives by a strain of Claviceps paspali Stevens and Hall in submerged Culture. Nature 187: 238 (1960).
- A. Smith and G. M. Timmis: The Alkaloids of Ergot. Part III. Ergine, a New Base obtained by the Degradation of Ergotoxine and Ergotinine. J. Chem. Soc. 1932: 763.
- A. Hofmann, R. Brunner, H. Kobel, und A. Brack : Neue Alkaloide aus der saprophytischen Kultur des Mutterkornpilzes von Pennisetum typhoideum Rich. Helv. Chico. Acta 40: 1358 (1957).
- M. Abe, T. Yamano, Y. Kozu and M. Kusumcgo : Production of Alkaloids by Ergot Fungus parasitic on Elymus mollis Trin. J. Agr. Chem. Soc. Japan 29: 364 (1955).
- Unpublished results from the Research Laboratories for Pharmaceutical Chemistry, Sandoz Ltd., Basel (Switzerland).
- . A. Stoll, A. Hofmann und W. Schlientz : Die stereoisomeren Lysergole und Dihydro-lysergole. Helv. Chim. Acta 32 : 1947 (1949).
- W. A. Taber and R. A. Heacock: Location of Ergot Alkaloids and Fungi in the Seed of Rivea corymbosa (L.) Hall.f. "Ololiuqui". Can. J. of Microbiol. 8: 137 (1962).
- W. A. Taber, R. A. Heacock and M. E. Mahon: Ergot-Type Alkaloids in Vegetable Tissue of Rivea corymbosa Hall.f. Phytochem. 2: 99
(1963).
- W. A. Taber, L. C. Vining and R. A. Heacock : Clavine and Lysergic Acid Alkaloids in Varieties of Morning Glory. Phytochem. 2: 65 (1963).
- M. C. Perezamador and J. Herrin: Turbicoryn: a new glucoside obtained from the seeds of a sacred plant. Tetrahedron Letters 1960: 30.
- W. B. Cook and W. E. Kealand : Isolation and partial characterization of a glucoside from Rivca corymbosa (L.) Hall.f. J. Org. Chem. 27: 1061 (1962).
- H. Solms : Chemische Struktur und Psychose bei Lysergaure-Derivaten. Paxis 45: 746 (1956).
- H. Solms: Relationships between chemical structure and psychoses with the use of psychotoxic substances. J. Clin. Exp. Psychopath. and Quart. Rev. of Psychiat. Neurol. 17: 429 (1956).
- T. Yui and Y. Takeo : Neuropharmacological Studies on a New Series of Ergot Alkaloids. Jap. J. Pharmacol. 7: 157 (1958).
Download Our Free Psychedelic Healing Books






 Plate IX
Plate IX 
 Ergometrine (formula II), the alkaloid which is mainly responsible for the uterotonic hemostatic action of the ergot drug, could only recently be identified as one of the active principles of Ipomoea violacea."[20] Together with elymoclavine, it forms spot D in the thin layer chromatogram (Plate IX, right). The seeds of Rivea corymbosa either do not contain this compound or else only traces thereof. Instead, we found lysergol (formula VII) in the last mentioned seed, an alkaloid absent from the seeds of Ipomoea violacea. Lysergol was produced synthetically[21] in our laboratories (as were d-lysergic acid amide, d-isolysergic acid amide and ergometrine) before it was discovered as one of the active principles of a Mexican magic drug. The compounds corresponding to spots E and F are present in such small quantities that they have hitherto not been identified.
Ergometrine (formula II), the alkaloid which is mainly responsible for the uterotonic hemostatic action of the ergot drug, could only recently be identified as one of the active principles of Ipomoea violacea."[20] Together with elymoclavine, it forms spot D in the thin layer chromatogram (Plate IX, right). The seeds of Rivea corymbosa either do not contain this compound or else only traces thereof. Instead, we found lysergol (formula VII) in the last mentioned seed, an alkaloid absent from the seeds of Ipomoea violacea. Lysergol was produced synthetically[21] in our laboratories (as were d-lysergic acid amide, d-isolysergic acid amide and ergometrine) before it was discovered as one of the active principles of a Mexican magic drug. The compounds corresponding to spots E and F are present in such small quantities that they have hitherto not been identified.

